Background:
Acute myeloid leukemia (AML) is the most common acute leukemia in adults with a median age at diagnosis of 68 years. Standard curative treatment consists of intensive induction chemotherapy followed by consolidation, allogeneic stem-cell transplantation, or both. However, older patients may be ineligible for intensive chemotherapy due to impaired performance status or significant comorbidities. For those medically unfit, the combination of venetoclax plus a hypomethylating agent (VEN + HMA) can be used based on the results of the phase 3 VIALE-A clinical trial (PMID: 32786187). With a median follow-up of 20.5 months, azacitidine-venetoclax achieved superior OS than azacitidine monotherapy (14.7 versus 9.6 months; HR 0.66; 95% CI 0.52-0.85; P<0.001). The only cardiac event reported was atrial fibrillation in 5% of patients. Our retrospective study explores real-world clinical outcomes and new cardiovascular adverse events of patients treated with venetoclax plus either azacitidine, decitabine, or LDAC (VEN + LDAC) in the Veterans Health Administration (VHA).
Methods
Medical records of 198 veterans who were patients in the VHA and treated with VEN + HMA or VEN + LDAC between January 1, 2016, and December 31, 2021, were randomly selected and reviewed. Patients with a diagnosis of AML who received front-line therapy were included. Patients with acute promyelocytic leukemia were excluded. Overall survival (OS) was defined as the period between the date of diagnosis and the date of death from any cause and was estimated using the Kaplan-Meier method. Cardiovascular adverse events were characterized as those that occurred from the first dose until 30 days after discontinuation of treatment.
Results
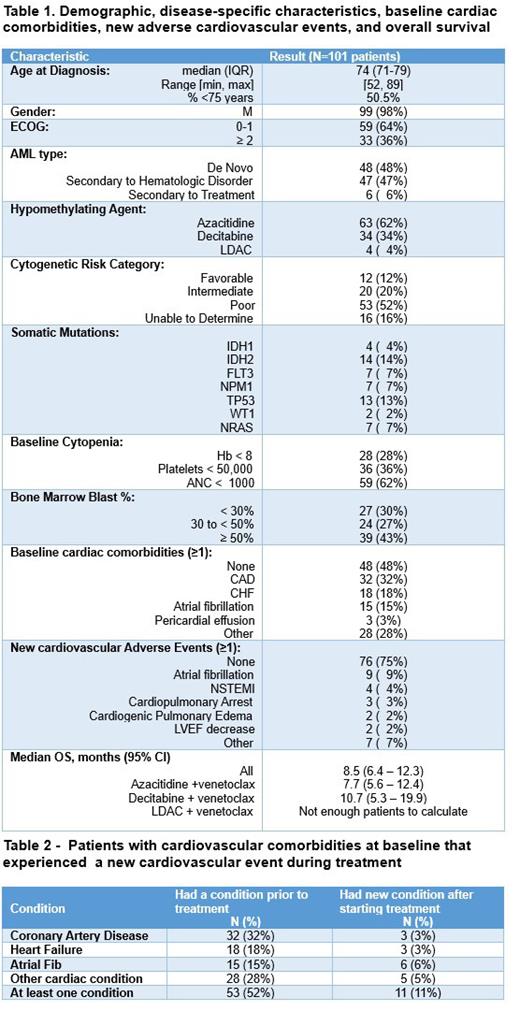
A total of 101 patients were included. The median age at diagnosis was 74 years, 98% were male, 48% presented with de novo AML, 64% had an Eastern Cooperative Oncology Group performance-status score ≤ 1, and 52% had poor cytogenetic risk according to the European LeukemiaNet (ELN) 2017 stratification. Azacitidine was used in 62% of patients, decitabine in 34%, and LDAC in 4%. Based on the common terminology criteria for adverse events (CTCAE) version 5, 28% of the cohort had grade ≥3 (≥G3) anemia, 36% ≥G3 thrombocytopenia, and 62% ≥G3 neutropenia. The median OS was 8.5 months (95% CI:6.4-12.3). Those who received azacitidine had an OS of 7.7 months (95% CI: 5.6 - 12.4) versus 10.7 with decitabine (5.3 - 19.9), however the difference was not significant (p=0.29). Twenty-five percent of patients experienced at least one new cardiovascular adverse event. Of those, 11% already had a cardiovascular comorbidity at baseline. The most common adverse events were atrial fibrillation in 9%, non-ST elevation myocardial infarction in 4%, cardiopulmonary arrest in 3%, cardiogenic pulmonary edema in 2%, and LVEF decrease in 2%.
Conclusion
This is the first study to analyze the OS and the array of new cardiovascular events of patients with AML treated with VEN + HMA or VEN + LDAC in the VHA. Our national retrospective cohort study showed an inferior median OS and more new adverse cardiovascular events in patients treated with VEN + HMA than what was seen in the VIALE-A study. This is likely due to the clinical design of the phase 3 trial. Furthermore, we had a higher percentage of patients with secondary AML and poor cytogenetic risk which are known adverse prognostic factors. The limitations of this study include its retrospective and observational nature with data that was created for patient care, not research, and might contain missing information. Besides, the VHA sites are exclusively located in the United States and the cohort is mainly composed of male patients which is not fully applicable to the general population. Further studies are needed to determine the real-world OS and profile of cardiovascular adverse events of venetoclax combination therapy.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal